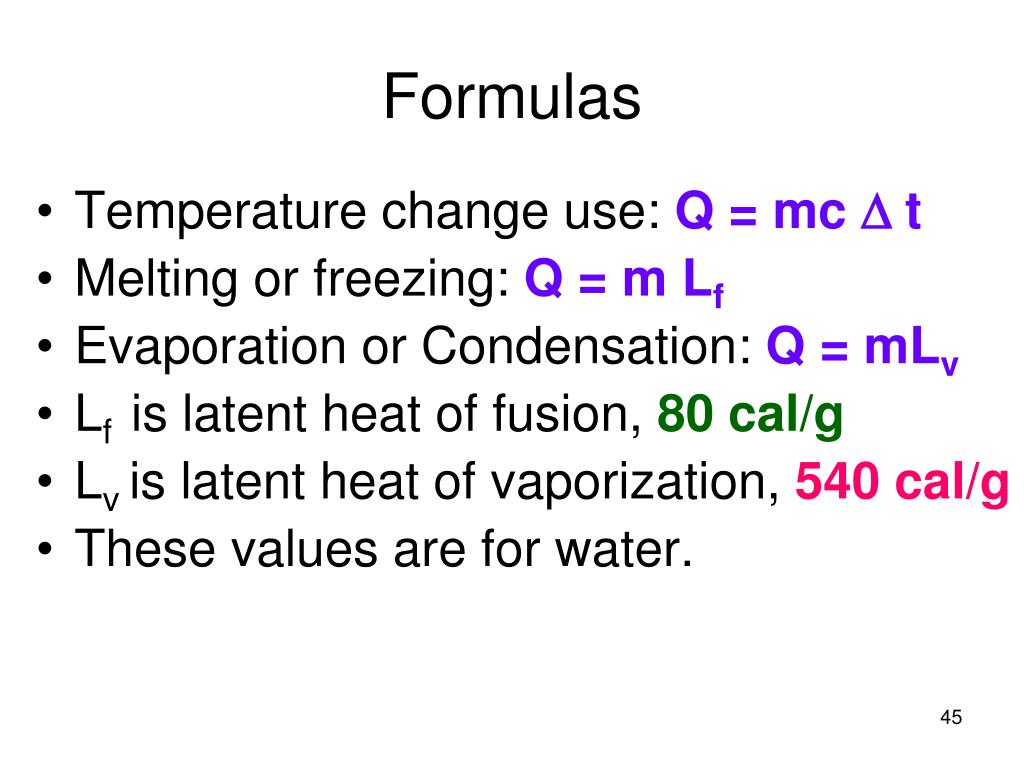
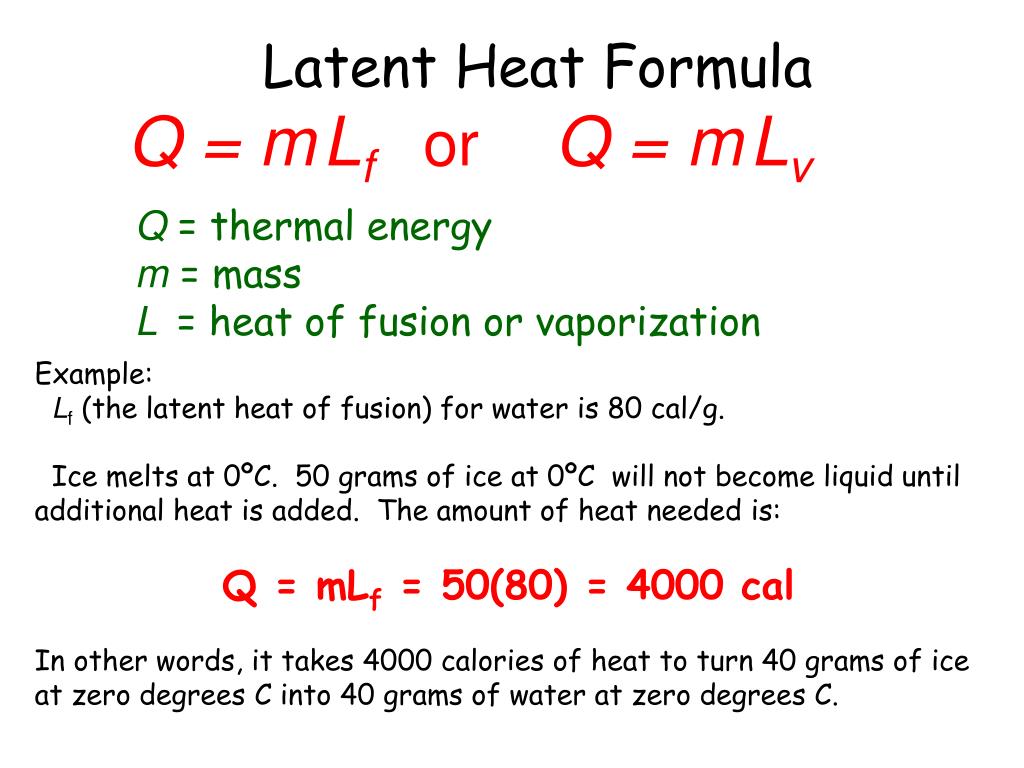
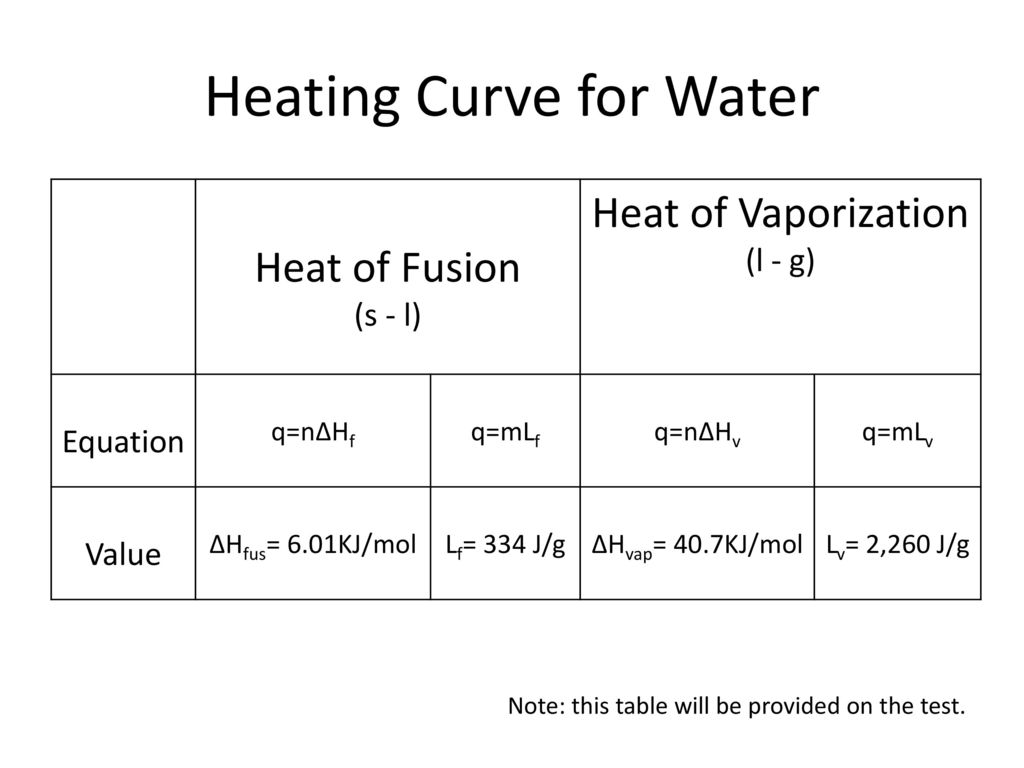
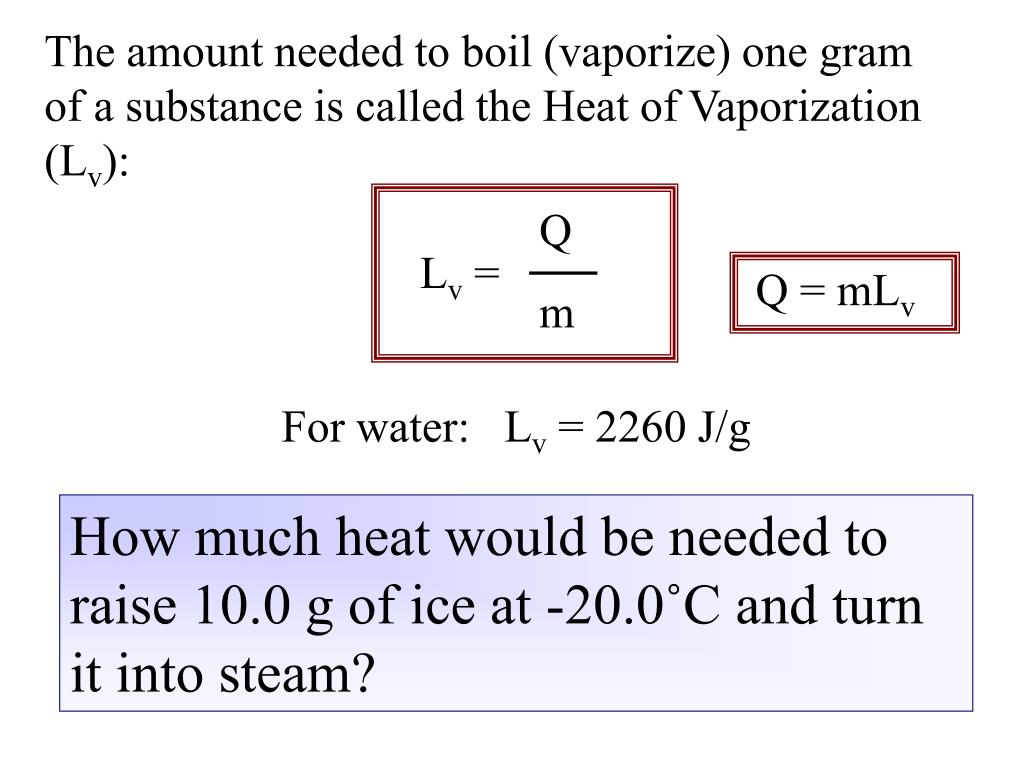
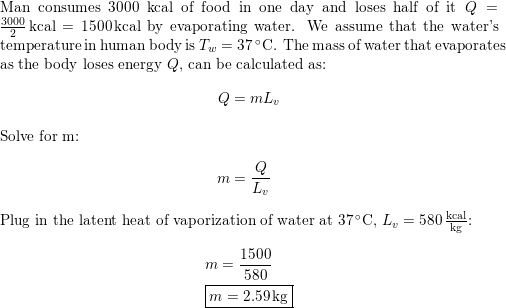HEAT EXCHANGE The exchange of thermal energy is simply referred to as heat. If an object rises/falls in temperature it has gained/lost thermal energy. - ppt video online download
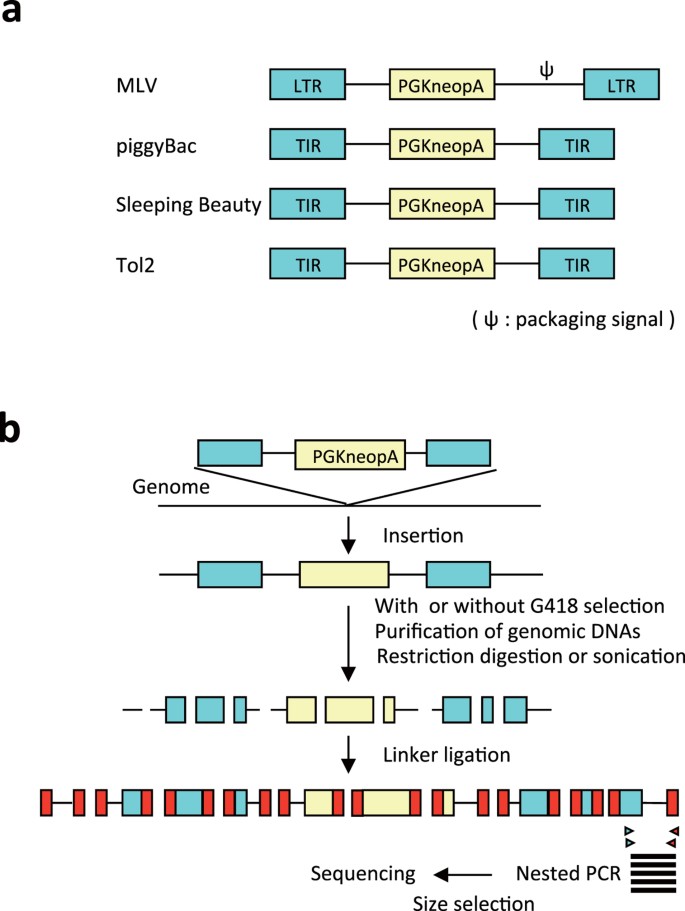
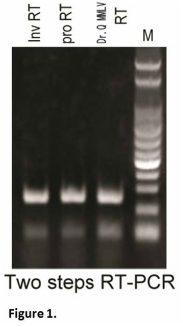
Chromatin states shape insertion profiles of the piggyBac, Tol2 and Sleeping Beauty transposons and murine leukemia virus | Scientific Reports
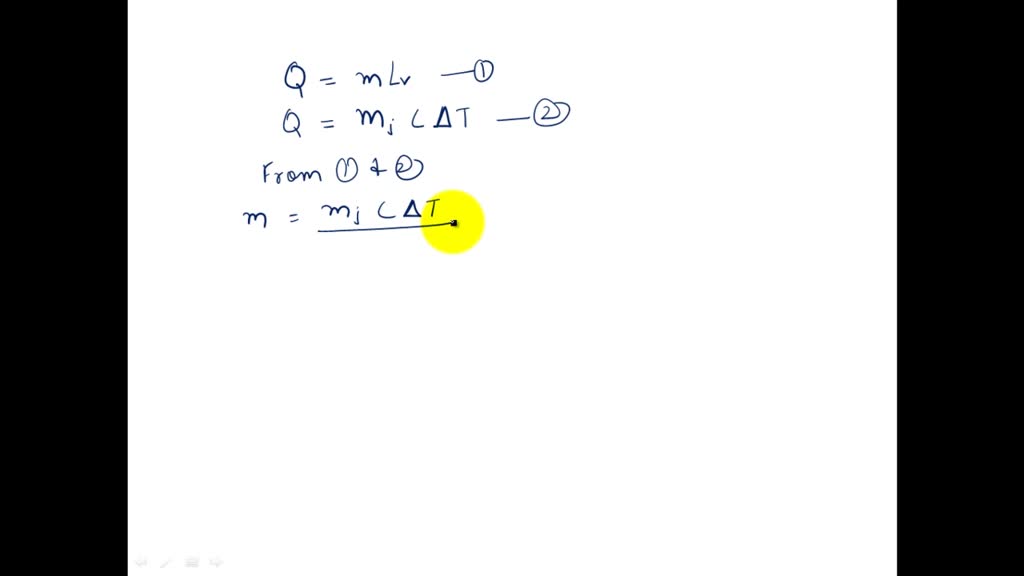
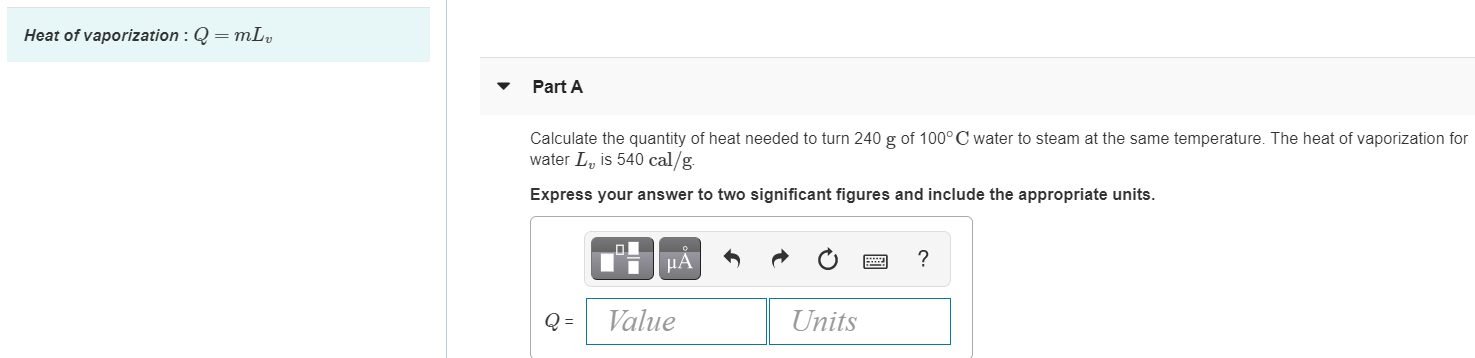
SOLVED:The latent heat of vaporization of H2 O at body temperature (37.0^∘ C) is 2.42 ×10^6 J / kg . To cool the body of a 75-kg jogger [average specific heat capacity =

Amazon.com: Soundsulate™ 1 lb Mass Loaded Vinyl MLV, Soundproofing Barrier 4' x 25' (100 sf) click for ADDITIONAL OPTIONS : Musical Instruments

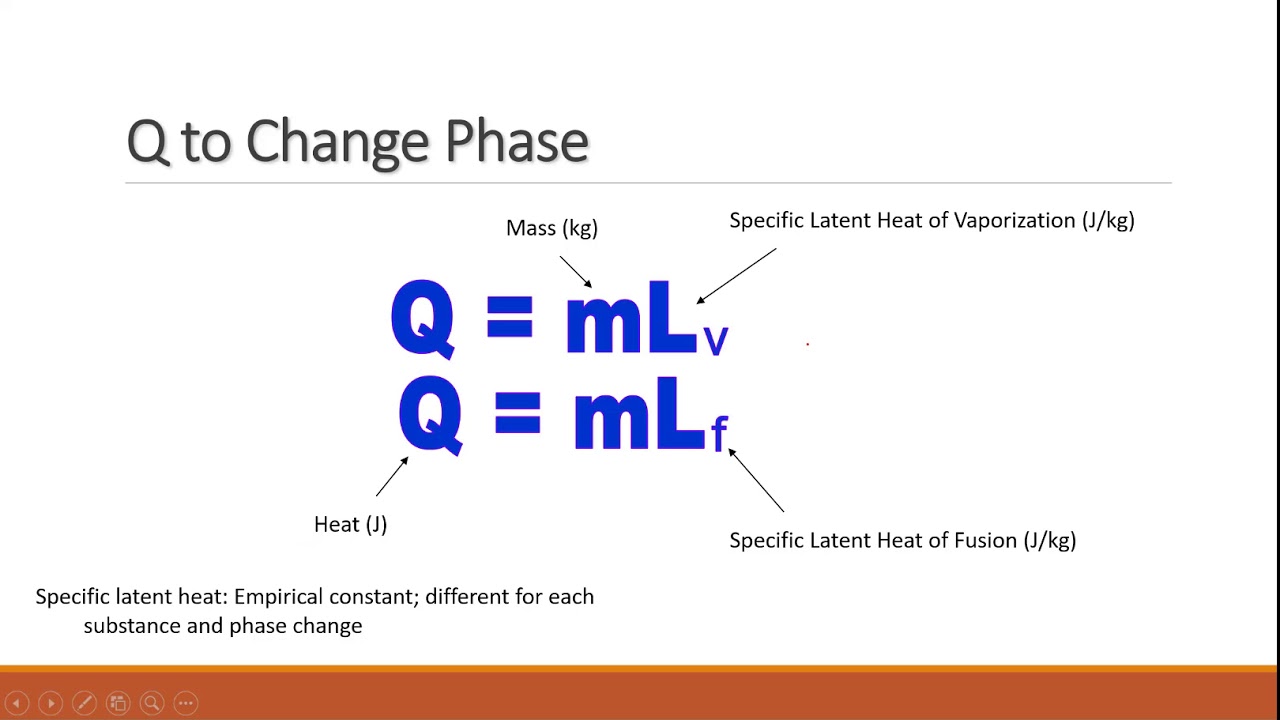
Heat required to convert one gram of ice at 0∘ C into steam at 100∘ C is given L stream =536 cal / gm A. 1 kilo calorieB. 100 calorieC. 0.01 kilo calorieD. 716 calorie